Biomaterials | Professor Zeng Yuanshan's Joint Team Designs and Synthesizes Peripheral Nerve Tissueoids for Repairing 1cm Sciatic Nerve Defects
In recent years, with the development of synthetic biology technology, there have been reports of using synthetic brain organoids, spinal cord organoids, and spinal cord-like tissues to repair central nervous system injuries. However, there is a lack of literature on the design and synthesis of organoids/tissues for repairing peripheral nerve defects. Therefore, this study follows the tissue engineering simulation design concept, using synthetic biology technology to load neurotrophin-3 (NT-3) overexpressing genetically engineered Schwann cells (SCs) within a decellularized optic nerve (DON) scaffold with natural uniform longitudinal channels. An artificial peripheral nerve tissue (peripheral nerve tissueoid, PNT) rich in laminin (LAM or LN) and NT-3 and other bioactive factors is manufactured to simulate the structure of peripheral nerve tissue in vitro. It is then transplanted to the site of sciatic nerve defect in an attempt to induce the longitudinal regrowth of axons in neurons expressing LAM or LN receptors and NT-3 receptors, restoring the function of the sciatic nerve and exploring the mechanism by which PNT grafts induce sciatic nerve regeneration.
To verify the above hypothesis, the joint team of Professor Zeng Yuanshan, Professor Xu Yangbin and Deputy Chief Physician Zhu Zhaowei from the First Affiliated Hospital of Sun Yat-sen University, Deputy Chief Physician He Bo from the Third Affiliated Hospital, and Deputy Researcher Li Ge from Guangdong Provincial People's Hospital transplanted PNT at the site of sciatic nerve defect. The results showed that PNT transplantation based on synthetic biology technology can repair the structure of the sciatic nerve defect and restore the sensory and motor functions of the paralyzed limb. This may involve inducing the interaction between NT-3 and its receptor TrkC cells, as well as the interaction between laminin and its receptor integrin αVβ6 axons, which play a role in chemotaxis, adhesion, and intrinsic drive mechanisms in promoting the targeted regeneration of neuronal axons within the PNT.
The results of testing the PNT showed that the unique bioactive matrix components LAMC3 or LN on the longitudinal channel walls of DON can form an NT-3-enriched area by binding with NT-3 secreted by genetically engineered Schwann cells (NT-3-SCs). The NT-3-SCs located in the PNT can also bind to the self-secreted NT-3 through their cell surface NT-3 receptor TrkC: on the one hand, it promotes the better colonization of NT-3-SCs in the NT-3-deposited area, forming a dynamic bioactive niche of PNT; on the other hand, the interaction between NT-3 and TrkC (ligand and receptor) further activates NT-3-SCs to better survive and secrete various neurotrophic factors, forming a microenvironment and structural basis for inducing the regrowth of neuronal axon bundles.
Transplanting the above PNT into the sciatic nerve defect site of rats, the animals survived for 8 weeks postoperatively. Through behavioral testing of the animals and detection of nerve-related tissues and functions, the results showed that the sciatic nerve index, limb rotation angle, and mechanical pain sensitivity test values of the PNT transplantation group were close to those of the autologous peripheral nerve transplantation group, with significant improvement compared to other transplantation control groups. Nerve-related tissue detection showed that compared with other transplantation control groups, the PNT transplantation group could promote the faster passage of regenerating axons across the sciatic nerve defect/transplant site to the target tissue and re-form myelin sheaths, thereby improving the sensory and motor functions of target skin and muscle tissues that had lost nerve innervation. Anterograde and retrograde tracing of nerve tissues indicated that regardless of whether in the PNT graft or at the distal end of the sciatic nerve defect/transplant site, adeno-associated virus (AAV) labeled dorsal root ganglion (DRG) neurons and their regenerated calcitonin gene-related peptide-positive axons could specifically express TrkC.
Transcriptome analysis of DRGs innervating the sciatic nerve defect/transplant side of the hind limb in the PNT transplantation group and the simple DON transplantation group revealed the potential molecular mechanisms of neuronal axon regeneration. The results revealed that the PNT graft provides a biophysical bridge at the sciatic nerve defect/transplant site to induce DRG neuronal axon regeneration. The NT-3 enriched on the longitudinal channel walls of the PNT by the genetically engineered Schwann cells activates DRG neurons and their axons to express the receptors TrkC and integrin αVβ6 (Integrin αVβ6) that combine with them, thereby activating the PI3K/AKT signaling pathway related to the intrinsic drive of neurons and enhancing the efficacy of long-distance axon regeneration.
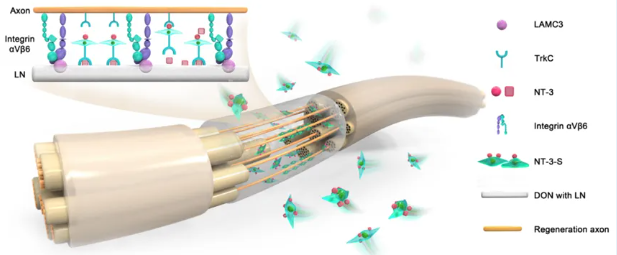
Schematic diagram shows: (1) LN or LAMININ on the PNT channel wall can bind with NT-3 to form an NT-3-enriched area, which is conducive to the colonization and growth of genetically engineered Schwann cells (NT-3-S) expressing TrkC and forming a niche for regenerating axons to form myelin sheaths on the PNT. (2) The continuous secretion of NT-3 by the PNT can promote the extension of TrkC-expressing neuronal axons to the sciatic nerve defect/transplant site, which may involve the interaction between NT-3 and TrkC as well as LAMININ and integrin αVβ6.
Original Article Link: //pubmed.ncbi.nlm.nih.gov/39531746/


