Cell Reports | Dong Junchao Team Unveils the Molecular Mechanism of Deubiquitinating Enzyme USP7 Collaborate TGF-b in Regulating Antibody Production
Chromatin loop extrusion is a fundamental mechanism for various molecular events such as chromosome packaging, separation, DNA repair, and RNA transcription. Recent studies have shown that cohesin-mediated loop extrusion plays a significant role in antibody diversification by promoting V(D)J recombination in developing B cells and class switch recombination (CSR) in mature B cells. The loop extrusion model suggests that during CSR, cohesin-mediated loops can extrude the IgH locus into loops, mediating the alignment, breakage, and joining of Sm and downstream transcriptionally activated S regions at the CSR centers, with the downstream Cx replacing the Cm exon, allowing the antibody to switch from IgM to other subtypes (IgG, IgE, or IgA, etc.) to exert different effector functions. Studies have shown that non-CTCF factors, including transcription, can act as barriers to regulate loop extrusion. However, how transcription regulates loop extrusion in the context of CSR is still unclear.
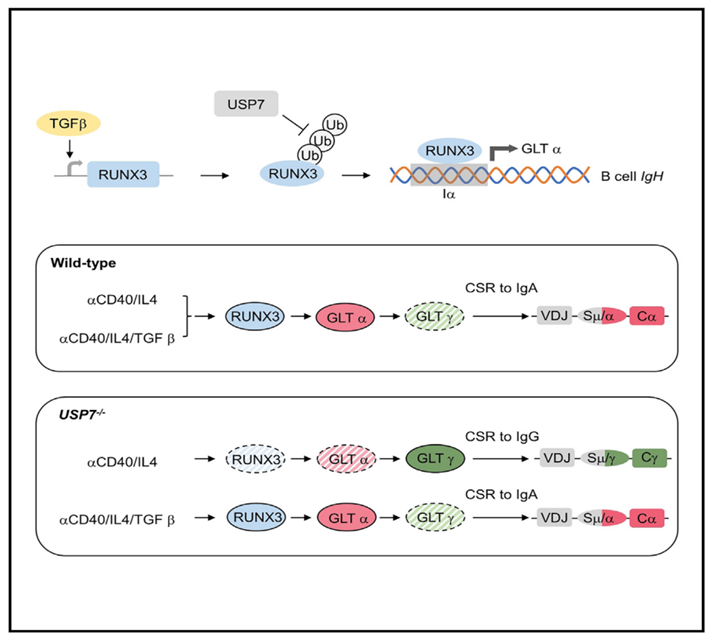
Recently, Professor Dong Junchao's team from Sun Yat-sen University's School of Medicine, in collaboration with Professor Chen Chun's team from the Seventh Affiliated Hospital of Sun Yat-sen University, published an article titled "USP7 promotes IgA class switching through stabilizing RUNX3 for germline transcription activation" in Cell Reports. The study indicates that USP7 and TGFb control the specificity of the CSR process by regulating the movement of cohesin-mediated extrusion loops towards the upstream S region by modulating Sa germline transcription activity.
In this study, the team constructed USP7-related mutant cells on the classic CH12 cell model. Compared with wild-type cells, under a-CD40/IL-4 stimulation, USP7-deficient cells showed significantly reduced Sa germline transcription (GLT) and CSR, while Sg GLT and CSR levels significantly increased. After adding TGFb to the above cells, the levels of Sa GLT and CSR in USP7-deficient cells significantly increased, while Sg GLT and CSR were significantly inhibited. The negative correlation between Sa and upstream Sg transcription and class switching suggests that Sa transcription can act as a barrier, inhibiting loop extrusion towards the upstream Sg region. This speculation was confirmed by 4C-Seq, indicating that USP7-mediated Sa germline transcription is crucial for Sa/CSR center interaction, and TGF-b compensates for the loss of interaction caused by USP7 knockout.
So, how do USP7 and TGFb regulate Sa transcription? By detecting the impact of USP7 on the protein levels of several transcription factors bound to the Ia promoter region, the research team found that USP7 can promote RUNX3 protein expression by interacting with the transcription factor RUNX3. USP7 can stabilize RUNX3 protein through its deubiquitinating enzyme activity, and TGFb can efficiently induce RUNX3 transcription, returning RUNX3 protein levels in USP7-deficient cells to wild-type levels.
In summary, this study, using a cell model where transcriptional activity can be inhibited and reversed, confirms the importance of the loop extrusion model for the production of B cell antibody region germline transcription, the three-dimensional conformation of the antibody gene locus, and the CSR process. It also discovers the regulatory effects of the TGF-b signaling pathway and the deubiquitinating enzyme USP7 on the transcription factor RUNX3 at both transcriptional and post-translational modification levels, a regulatory mechanism that may also apply to other non-B cell scenarios involving TGF-b and USP7. Furthermore, given USP7's specific promotion of IgA rather than IgG antibodies, small molecule inhibitors targeting USP7's deubiquitinating enzyme activity may have potential therapeutic effects in IgA-induced inflammation, autoimmune, and other immune disorders.
Professor Dong Junchao from Sun Yat-sen University's School of Medicine and Professor Chen Chun from the Seventh Affiliated Hospital of Sun Yat-sen University are the co-corresponding authors; team members Dr. Zhao Bo and Dr. Xia Zhigang are the co-first authors. Professor Zhang Xuefei from the Peking University Biomedical Frontier Innovation Center, Researcher Zhu Chengming from the Seventh Affiliated Hospital of Sun Yat-sen University, Professor Liang Jintang from the Chinese University of Hong Kong, and Deputy Chief Physician Huang Junbin from the Seventh Affiliated Hospital of Sun Yat-sen University also made significant contributions to this research.
Original Article Link: //doi.org/10.1016/j.celrep.2024.114194